by Rachel Gore
When patients leave a doctor’s office with a new prescription, they expect that prescription to make them feel better. And while no medication comes without any risks, patients expect to be properly informed of what those risks are.
Unfortunately, that’s not always the case, and in some cases, medication can end up doing more harm than good. This seems to be the case for some patients prescribed Elmiron, a drug used to treat pain and discomfort associated with a bladder condition called interstitial cystitis.
Now, plaintiffs around the country are filing lawsuits against the drug manufacturer Janssen Pharmaceuticals for failing to properly warn consumers about Elmiron’s link to a unique and serious condition that causes eye damage.
Here is everything you need to know about the medication Elmiron and the research linking it to vision loss:
What is Elmiron?
Elmiron is the brand name for the drug pentosan polysulfate sodium (PPS). It was released in 1996 by the manufacturer Janssen Pharmaceuticals and is the only Food and Drug Administration (FDA) approved medication to treat interstitial cystitis. Elmiron has no generic alternative.
Interstitial cystitis (IC), or painful bladder syndrome, is a chronic condition that causes bladder and pelvic pain or pressure and a persistent and urgent need to urinate. It can massively impact the quality of a person’s daily life.
To put that impact into perspective, the average adult urinates around four to 10 times a day. But according to the University of Washington Department of Urology, individuals with severe cases of interstitial cystitis can urinate as many as 60 times per day, including multiple times throughout the night that disrupt sleep. The level of pain experienced ranges from mild to agonizing.
In general, interstitial cystitis affects more women than men. The Office on Women’s Health estimates that between 3 million and 8 million women in the U.S. alone are living with the condition. For men, the number is between 1 million and 4 million. However, this may be an underestimate, as IC in men is often misdiagnosed as other disorders that affect the bladder.
What is Pigmentary Maculopathy?
While Elmiron is often effective in relieving the symptoms of painful bladder syndrome, a growing body of research has linked it to the development of a serious eye condition called maculopathy.
Maculopathy affects the macula, which is in the back of the eye’s retina. Although it measures a mere 5 millimeters across, it is largely responsible for central vision, color vision and seeing fine detail.
Pigmentary maculopathy is a unique form of maculopathy specifically associated with chronic exposure to Elmiron. In other words, it is an eye condition like no other that is associated exclusively with long term Elmiron exposure.
Patients with pigmentary maculopathy develop pigmented deposits that resemble specks in the macula. They can experience gradually worsening vision problems and visual distortions such as straight lines seeming bent, blurriness and well-defined blurry or black spots in the field of vision.
While a vast majority of individuals with maculopathy don’t go completely blind, those with severe cases can lose their central vision and become classified as legally blind. Elmiron-induced vision damage starts off looking like a tiny drop of black ink in their field of vision for some patients. Over time, that drop expands and increasingly affects their quality of life.
Some Elmiron patients “go from clear vision but a painful bladder to improvement in the bladder pain but [they] can’t see anything in front of them, can’t drive a car, can’t perceive distances and can’t see the people they love and care around them,” said Tobi Millrood, a partner at the Pennsylvania law firm Pogust Millrood, LLC and president of the American Association for Justice.
For those patients, the damage caused by Elmiron far outweighs the benefits. “As painful as the bladder can be from interstitial cystitis, I am confident that 100% of people would have a healthy vision and a painful bladder rather than being pain free in their bladder and not being able to see. It’s just not worth the risk,” Millrood added.
Symptoms of Pigmentary Maculopathy
Pigmentary maculopathy is a form of maculopathy specifically linked to the use of Elmiron. Here is a list of its most common symptoms:
- Blindness
- Blurred vision
- Dark spots
- Difficulty reading
- Difficulty adjusting to darkness
- Dull or muted colors
- Eye pain or discomfort
- Metamorphopsia, or vision distortion that makes straight lines look curved
- Paracentral Scotoma, or vision loss or blurriness in the vision that is slightly off-center
A Deep Dive into Research Findings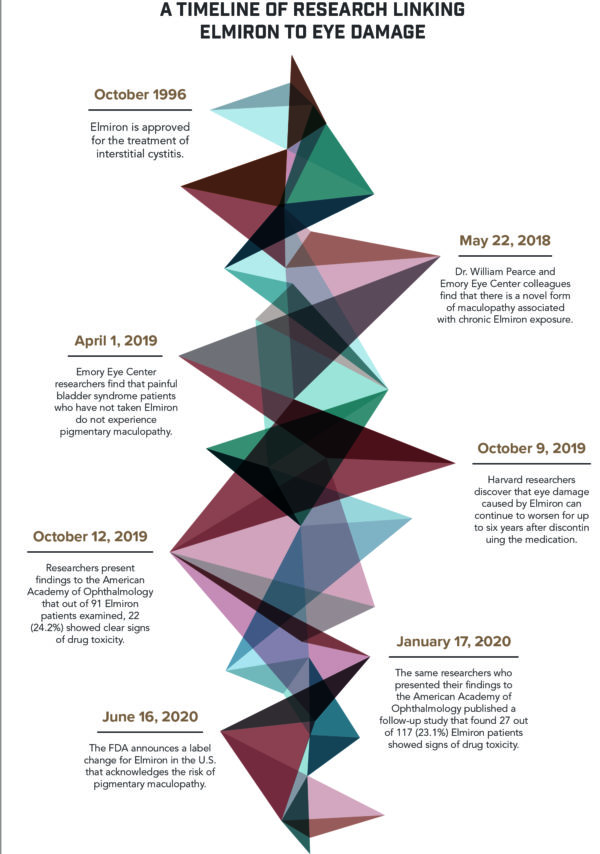
Between 1997 and 2019, Janssen Pharmaceuticals filed over 100 adverse event reports regarding eye-related injuries caused by Elmiron. Beyond these reports, there is also a growing body of research linking Elmiron to pigmentary maculopathy.
The Emory Eye Center Findings
In May 2018, Dr. William Pearce and colleagues at the Emory Eye Center published an article in Ophthalmology, the journal of the American Academy of Ophthalmology, describing a “novel and possibly avoidable maculopathy associated with chronic exposure to PSS.” Patients reported symptoms including having difficulty reading and prolonged dark adaptation.
As one of the first medical journal studies linking Elmiron to vision damage, the Pearce study was a watershed moment that triggered widespread awareness of this risk. Shortly after its publication, a wave of plaintiffs across the country began retaining legal counsel for their injuries related to Elmiron.
In April 2019, The Emory Eye Center published an additional study in The Journal of Urology, finding that patients with a history of painful bladder syndrome who did not take Elmiron did not have the pigmentary maculopathy present in patients who did take it. The researchers recommended that Elmiron patients with symptoms stop taking the medication and Elmiron patients without symptoms undergo retinal imaging.
Kaiser Permanente Findings
In October 2019, Drs. Rob Vora, Amar Patel and Ronald Melles presented their research findings at the Annual Meeting of the American Academy of Ophthalmology after analyzing detailed images of Elmiron patients’ eyes. In total, they analyzed the eyes of 91 patients who had each taken an average of 5,000 Elmiron pills over the course of 15 years.
The ophthalmologists found that 22 of the 91 patients, or 24.2%, showed definitive signs of drug toxicity—when too much of a medication accumulates in the bloodstream and causes negative health impacts. How drug toxicity presents itself depends on both the drug taken and the dosage.
In the case of Elmiron, the researchers found that Elmiron drug toxicity resulted in damage to the retina. They also found that the rate of drug toxicity rose with the amount of Elmiron consumed. Drug toxicity was present in 42% of patients taking 1,500 grams or more of Elmiron, but only 11% of those taking 500 to 1,000 grams.
The three researchers published a follow-up study in January 2020 looking at the data of 117 patients who took Elmiron for painful bladder syndrome. Among them, 27 showed definitive signs of maculopathy. Like their previous findings, patients who took more than 1,500 grams had the highest risk of drug toxicity.
Harvard Medical School Department of Ophthalmology Findings
In November 2019, Drs. Rachel M. Huckfeldt and Demetrios Vavvas of the Harvard Medical School Department of Ophthalmology published a study revealing that retinal damage can progress years after the discontinuation of Elmiron.
They made this discovery by analyzing the eyes of a Harvard Medical School clinic patient who came in due to vision problems. Prior to her initial visit, the 62-year-old woman had taken low doses of Elmiron for 18 years.
Clinicians found that the woman had pigmentary changes in her retina. A follow-up examination six years after the initial visit revealed that her retinal damage had significantly increased despite the fact that she was no longer taking Elmiron.
Northwestern University Department of Ophthalmology Findings
In February 2021, Neil S. Kalbag, Nenita Maganti, Alice T. Lyon and Rukhsana G. Mirza from Northwestern University published a study in Clinical Ophthalmology offering additional evidence of the risk Elmiron poses. The study analyzed medical records data from a total of 131 patients who were exposed to Elmiron and seen at an ophthalmology clinic between 2002 and 2019. Among the 40 patients who had received eye imaging, 12.5% showed signs of Elmiron maculopathy. Of the remaining 91, five had pigmentary changes described in their exam reports.
While these are not the only studies on Elmiron and maculopathy, they are key examples among a growing body of research pointing to this link.
Janssen’s Delay in Updating the Elmiron Label
With mounting evidence that Elmiron use can cause maculopathy, the European Medicines Agency (EMA) demanded in summer 2019 that the medication’s label be updated to inform consumers of the risk. In October 2019, Health Canada demanded the same. While Janssen Pharmaceuticals met these demands, it did not change the label in the United States to reflect the risk until June 26, 2020.
In an announcement made on that date regarding the change, the FDA stated that “[p]pigmentary changes in the retina, reported in the literature as pigmentary maculopathy, have been identified with the long-term use of ELMIRON®. Although most of these cases occurred after three years of use or longer, cases have been seen with a shorter duration of use. While the etiology is unclear, cumulative dose appears to be a risk factor.”
For many consumers, the warning came too little, too late. According to Gabe Magee, an attorney at Pogust Millrood, many plaintiffs filing Elmiron lawsuits against Janssen feel that their eye damage was preventable.
“If you look at the label now, it recommends that people who take this drug get regular eye screenings, that they start with a baseline eye exam before they take [Elmiron] and then after that continue to get regular screenings. If something that simple had just been instituted by Janssen, a lot of our clients feel like they could have avoided these issues and they wouldn’t be suffering from something that’s permanent,” he said.
Another purpose of these lawsuits is to prevent things like this in the future… [Lawsuits] get the word out to other potential consumers about this drug, but [they] can also have a potential effect for other drugs that are out there from drug companies.
– Gabe Magee, Attorney
Lawsuits Against Janssen are Mounting
Since 2018, consumers who developed eye damage after taking Elmiron have reached out to legal counsel around the country. It’s expected that, in time, hundreds to thousands of affected individuals will file lawsuits against Janssen Pharmaceuticals for their injuries. Elmiron lawsuits claim that Janssen failed to warn the public about risks that the medication causes.
“Not until recently, neither physicians nor patients who were prescribing or ingesting the Elmiron were made aware of the risks associated with it, which is why we think there’s going to be a very strong liability case, particularly because Janssen was certainly on either actual or constructive notice. They either knew or should have known the risks based on a number of things that have been in medical textbooks and journals,” said Millrood.
Legal action against Janssen pertaining to Elmiron is still in the early stages. Currently, there are two main areas where cases are being litigated. In federal court, over 170 lawsuits are being consolidated into a multidistrict litigation overseen by the U.S. District Judge Brian Martinotti of the District of New Jersey. Cases are also mounting in the state of Pennsylvania, where Janssen is headquartered. It is possible that these could be consolidated in the future.
The cutoff for the discovery period—the pre-trial period in which parties can obtain evidence from the other parties—of the earliest filed case is July 2022. This means that the earliest trial will likely occur at the end of 2022 or in 2023. Until then, expect a growing number of plaintiffs to come forward to take legal action against Janssen.
Why Does This Matter?
Elmiron is the only FDA-approved drug on the market to treat interstitial cystitis. However, adverse event reports and a growing body of research have linked the medication to a serious eye condition called pigmentary maculopathy, which can result in severe vision loss and a dramatic decrease in quality of life.
Despite this evidence, the drug manufacturer Janssen Pharmaceuticals did not add a warning to Elmiron’s label regarding this risk in the United States until June 2020.
Research by William Pearce and associates at the Emory Eye Center published in 2018 was a turning point, triggering consumers who developed eye damage after taking Elmiron to file lawsuits against Janssen.
While only time will tell the outcome of these lawsuits, many consumers still have the opportunity to seek out legal counsel themselves to determine whether they have a case for compensation against Janssen after taking Elmiron. In the meantime, as long as medication continues to do damage, personal injury attorneys will be there to fight back.
“Another purpose of these lawsuits is to prevent things like this in the future… [Lawsuits] get the word out to other potential consumers about this drug, but [they] can also have a potential effect for other drugs that are out there from drug companies,” said Magee.







Leave A Comment